3 strategic uses of timelines for pharma projects success
Learn how to coordinate clinical trials, optimize resources, and prepare impactful presentations using free PowerPoint templates.
In the pharmaceutical industry, managing time-sensitive tasks isn't just a challenge, it’s a necessity. Each stage of drug development, from early discovery and preclinical testing to clinical trials and post-market surveillance, is tied to strict timelines and deadlines.
Add the pressure of managing stringent regulatory requirements, and the complexity can quickly escalate. Without effective time tracking, delays can ripple through the process, driving up costs and jeopardizing the whole development process.
The solution? A clear, structured timeline that helps keep projects on track and reduces stress, where complex information is presented accurately and in a simplified way. Breaking down each step of the process, setting achievable milestones, and tracking progress allows project managers to identify issues early.
Visual tools like timelines, Gantt charts, and roadmaps do more than just organize project processes, they make it easier to share updates, giving stakeholders a clear understanding of where things stand and what’s coming next.
In this article, we’ll look at how to use these tools in three time-sensitive scenarios: coordinating clinical trial phases, optimizing resource allocation in pharma projects, and preparing presentations for conferences and stakeholder updates. All with the help of free templates that you can download and customize to fit your next project presentation.
Use case 1: Clinical trial coordination and milestone tracking
Keeping timelines on track in clinical trials requires precise coordination. Deadlines must be met at each stage, from patient recruitment to data analysis, with specific requirements that need to stay on schedule to keep the project running.
In Phase I trials, for instance, the focus is on testing safety and determining the right dosage in small groups. Staying on track here means patient recruitment and dosing happen as planned so that safety assessments aren’t delayed.
Phase II shifts to testing the drug’s effectiveness and monitoring side effects, requiring careful planning to enroll enough patients and analyze interim data as scheduled.
By Phase III, the emphasis is on confirming the drug’s effects in a larger population and preparing for regulatory submission.
Setbacks at any phase can throw off the entire project timeline. Costs increase and progress is slow. A well-structured timeline can help track each step, reducing the risk of disruptions.
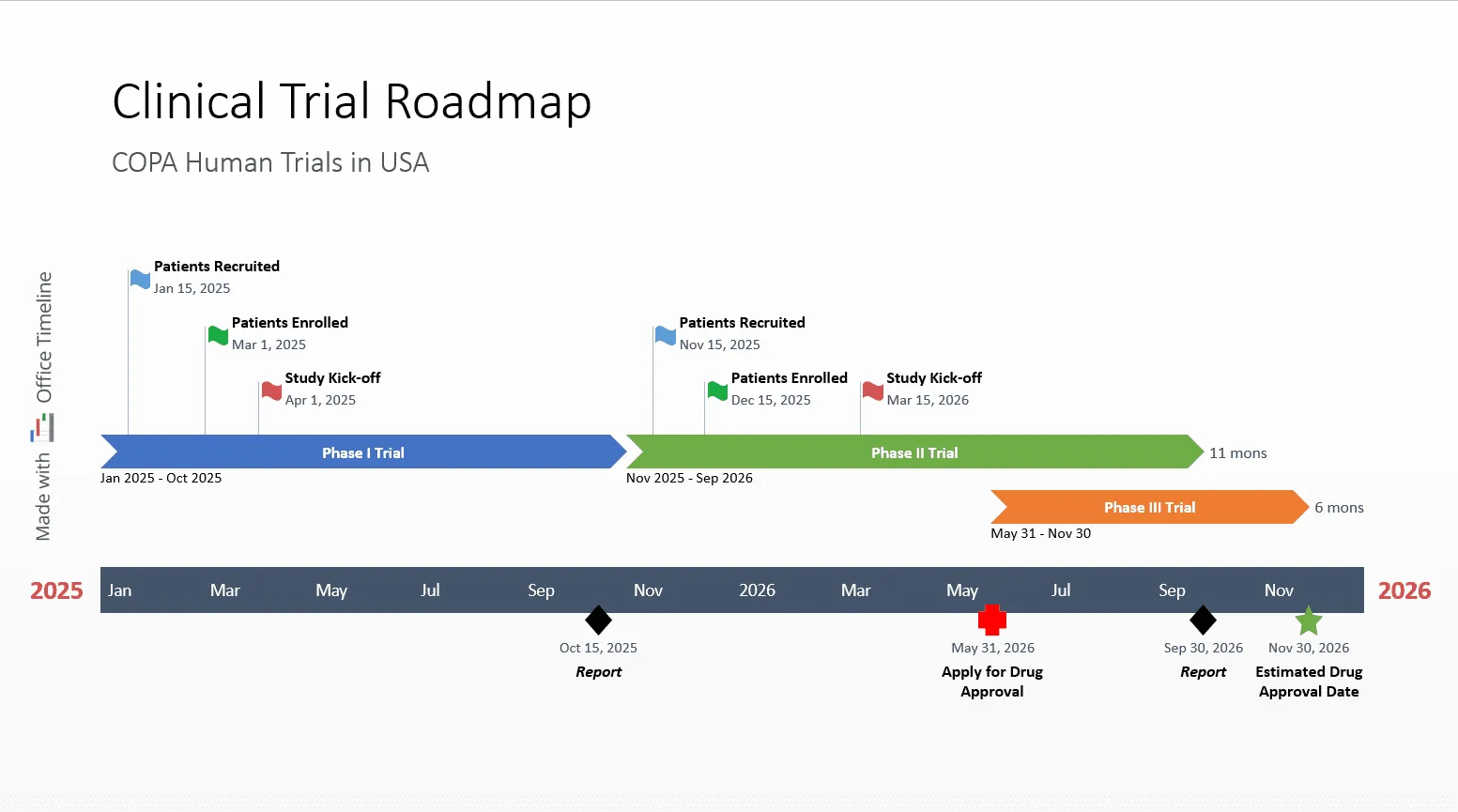
Take for example the clinical trial timeline template below, that shows an easy and practical way to track the progress across phases:
With a clear view of the milestones (patient recruitment, study start dates) and the deadlines, it’s easy to track the progress of the process and data collection and to follow each phase of the trial.
This way, the project manager can make sure that the clinical trial stays within the required timeframe. Potential problems can be spotted early, and steps can be taken to prevent them.
This file works as a template and can help you keep the clinical trial organized while saving you time and effort in planning and tracking tasks. Just download it for free and modify data as needed with the help of Office Timeline’s free 14-day trial. Or use the free trial add-in to create a clinical trial roadmap by importing your data from Excel or MS Project, or simply copy-paste it or add it directly into the PowerPoint wizard.
Prioritization and sequencing issues in clinical trials
Another pain point in clinical trial management is prioritizing multiple ongoing studies within a complex and resource-intensive portfolio. This is crucial because, as researcher Dr. Tony Kennedy shows in his book Pharmaceutical Project Management, the constant reprioritization of clinical trials can strain resources.
A common issue arises when resources are shifted to support a trial in crisis, which demands immediate attention and disrupts other projects, delaying them further. This creates a cycle of reactive management.
Reactive management refers to handling problems as they come up rather than anticipating and preventing them. Or simply put, responding to issues only after they’ve disrupted the project. This makes it difficult to stick to original plans and often leads to stress, delays, and higher costs.
Using a structured timeline to plan and track clinical trials can help break the cycle of reactive management. By visualizing each trial’s schedule, along with dependencies and potential conflicts, a project manager gets the perspective needed to make informed decisions.
A timeline can show at a glance the status of the clinical trial. The project manager then can easily see which studies require immediate attention and which can be delayed. This level of visibility can prevent last-minute changes and help minimize disruptions.
How? By preventing costly overlaps and improving resource use (like research staff and facilities).
The lack of clarity in prioritization or having too many priority levels can create confusion and inefficiency. As Tony Kennedy notes in his book Pharmaceutical Project Management, one company reportedly used as many as 40 priority levels. Obviously, these were difficult to track and frequently changed without notice. This led to constant disruptions, with resources frequently shifted between projects. The result? Delays and inefficiencies, especially for companies that handle unrelated studies for multiple clients.
The solution? Simplifying to just a few priority levels. A timeline maker can help organize trials using just a few clear priority levels. By visualizing each study’s schedule and dependencies, a project manager can better sequence trials and make informed decisions about which to advance and which to delay.
This reduces unnecessary resource switching and keeps the entire project portfolio more stable and manageable.
Use case 2: Pharma projects resource optimization and the risk of attrition
One of the most challenging aspects of managing pharmaceutical projects is resource allocation, especially given the high attrition rates in drug development. Tony Kennedy shows in Pharmaceutical Project Management that most pharmaceutical companies experience a significant drop in project numbers from preclinical phases to registration.
For instance, out of nine projects entering preclinical development, only one typically makes it to a new drug application (NDA). The highest attrition occurs in Phase 1 and Phase 2 trials, often due to lack of efficacy or safety concerns identified early. This highlights the need to carefully manage resources: keeping nonviable projects running too long can waste huge amounts of time and money, especially since later-phase trials are so costly.
To combat inefficiencies, using a structured timeline can help manage resources better by focusing on important decision points. Timelines can clearly show attrition risk and resource distribution. Inefficiencies can slow down your project and waste resources.
By plotting out each phase, project managers can anticipate where resource shifts may be needed, and pinpoint which projects are nearing important decision points. This visibility ensures resources are used wisely, and projects with potential get the support required to move forward.
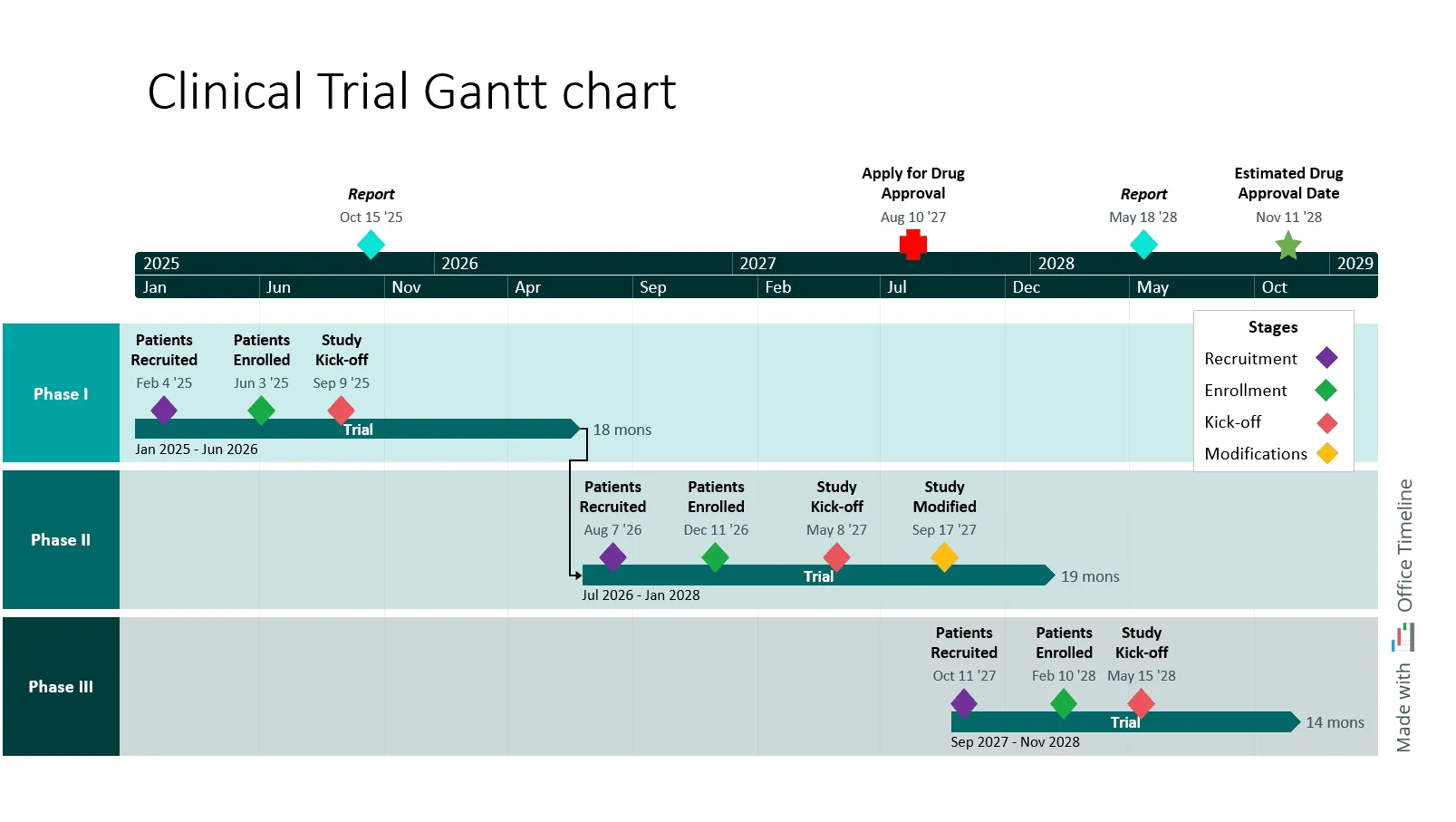
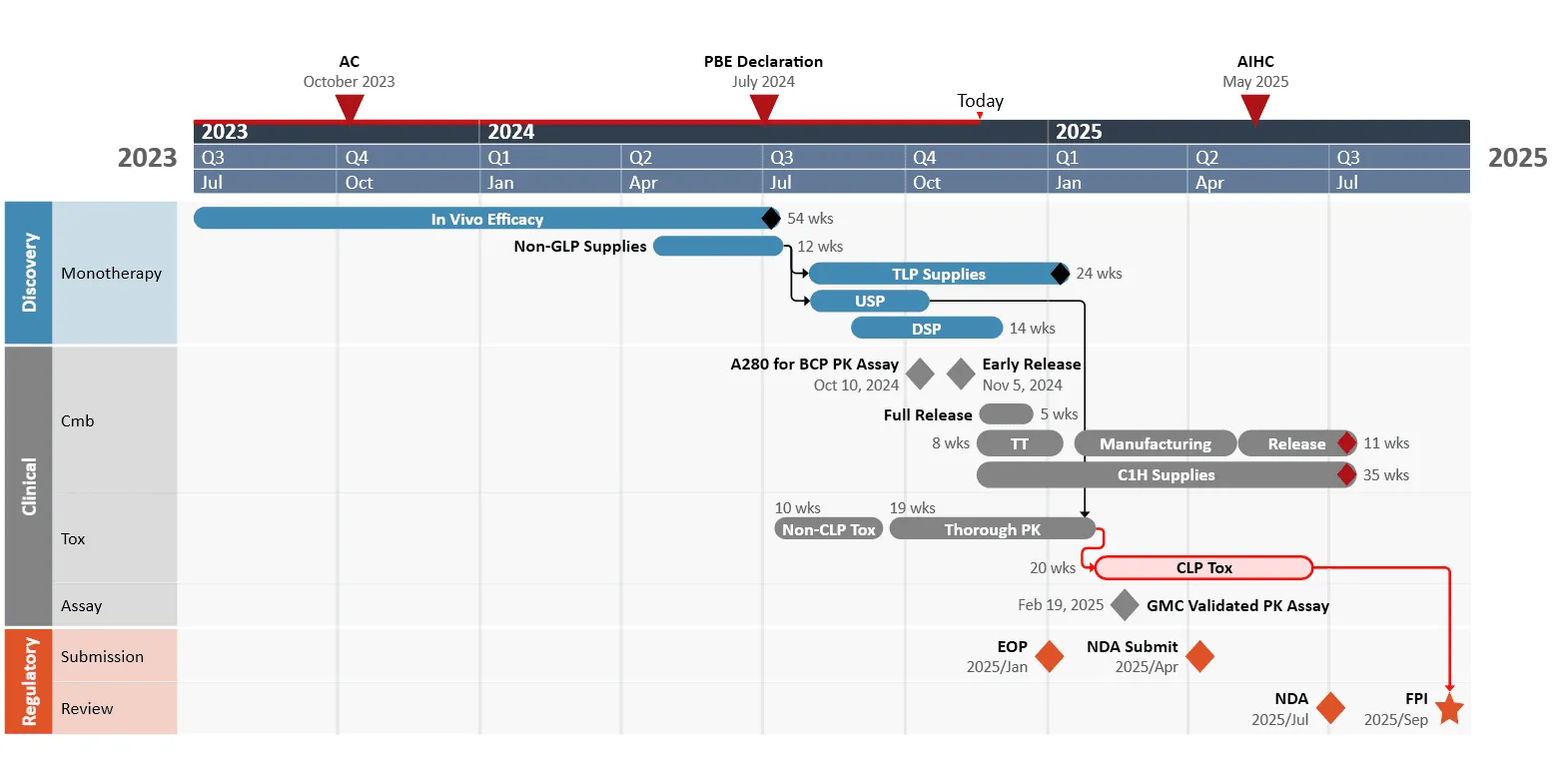
Visual timelines add value by allowing project managers to test different scenarios and simplifying the process of adjusting plans when a project shows signs of risk. For instance, the example timeline below shows the exact details of each stage and illustrates how a delay scenario compares to the planned schedule - easy to see where adjustments are needed.
This roadmap is organized using swimlanes to clearly separate each phase of development: Discovery, Clinical, and Regulatory. Each swimlane provides specific details relevant to that phase, making it easier to understand how tasks are structured and executed over time.
Milestones are marked along the timeline, serving as decision points that help evaluate progress and determine whether the project should continue or be adjusted. Additionally, project dependencies are mapped out, showing the sequence and interconnection of tasks. This setup allows for efficient resource management, better adaptability to changes, and reduces the risk of delays or unexpected costs.
This roadmap was created with Office Timeline, a professional PowerPoint add-in designed for creating clear and visually engaging timelines. Download Office Timeline’s free 14-day trial to quickly create your own tracking tool by adding your data into pre-built templates or build a timeline from scratch.
You can import data directly from Excel or MS Project or use the copy-paste feature to simplify the timeline creation process and keep your projects on track effortlessly.
Use case 3: Scientific presentations for pharma conferences, summits and congresses
In pharmaceutical research, the key to a strong presentation of project updates is clearly communicating intricate timelines to industry experts and potential investors. Condensing this information requires precision and a strategic focus to keep the audience engaged and informed.
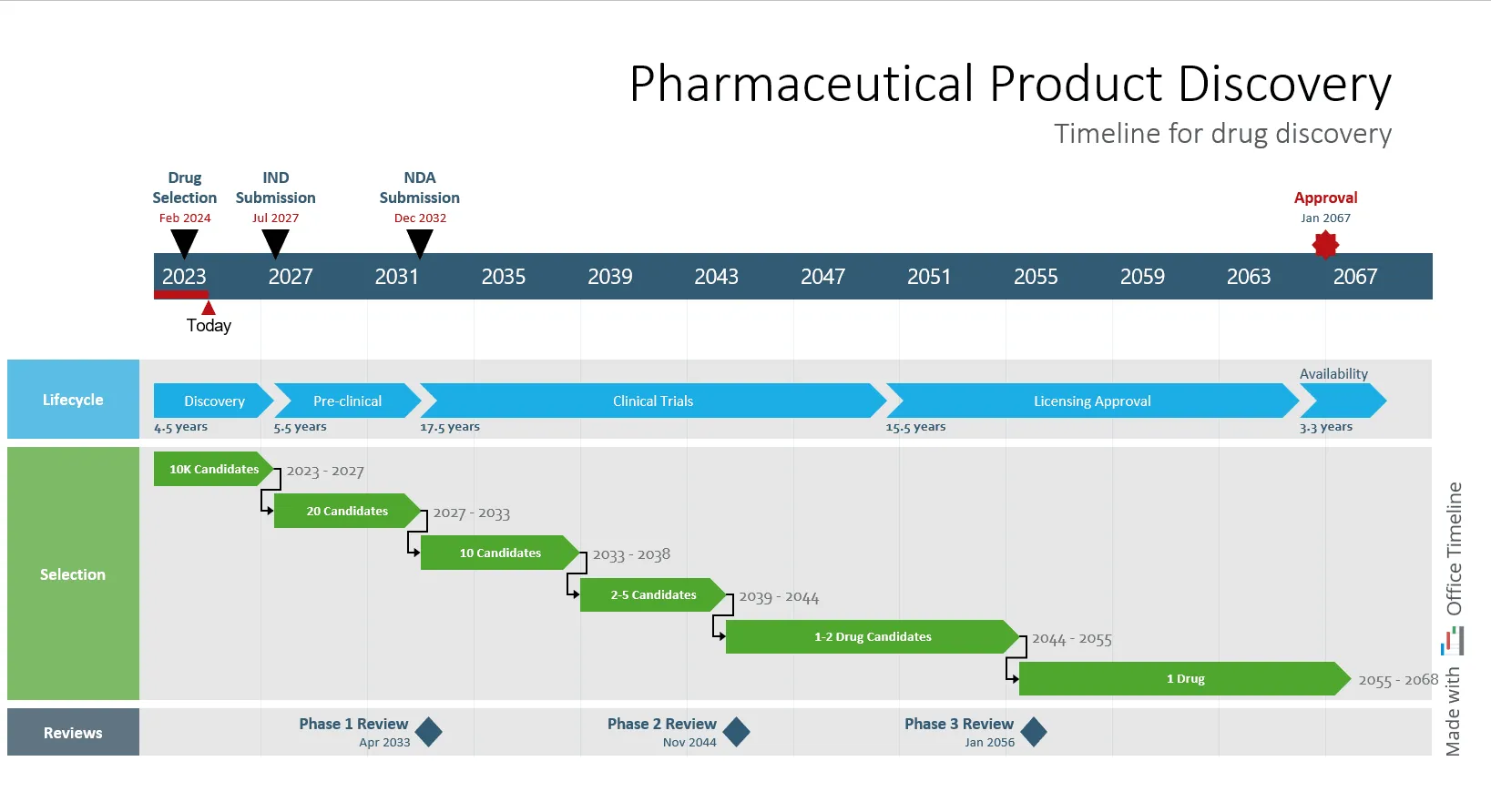
For instance, when presenting a drug development timeline, you may need to cover years of clinical research in a concise form. Such a development program may span sometimes even decades, involving multiple phases, with overlapping trials, specific features, deadlines and submission dates.
This task becomes even more challenging when you need to prepare for a presentation at pharma summit events, medical congresses, conferences, or symposiums, where presentation duration is limited, and PowerPoint is often the go-to tool for showcasing complex data.
So how do you do that? One solution to this challenge is using a professional tool like the Office Timeline add-in, designed specifically to simplify and enhance complex project visuals with minimum effort. With this software, you can transform dense project details into easy-to-read timelines that integrate seamlessly right into your PowerPoint presentation. You save considerable time and effort when preparing your visuals.
Best of all, you keep your audience engaged and interested throughout the presentation. Rather than overwhelming them with text-heavy slides, you present a visually organized timeline that stands out for its simplicity and clarity, making your message easy to follow and impactful:
Each milestone, from patient enrollment dates to regulatory submission targets or when the next steps are scheduled, is clearly marked. Your audience can grasp the progression and key events at a glance. They can understand the full scope and sequence of the drug development program and how each milestone fits into the development strategy.
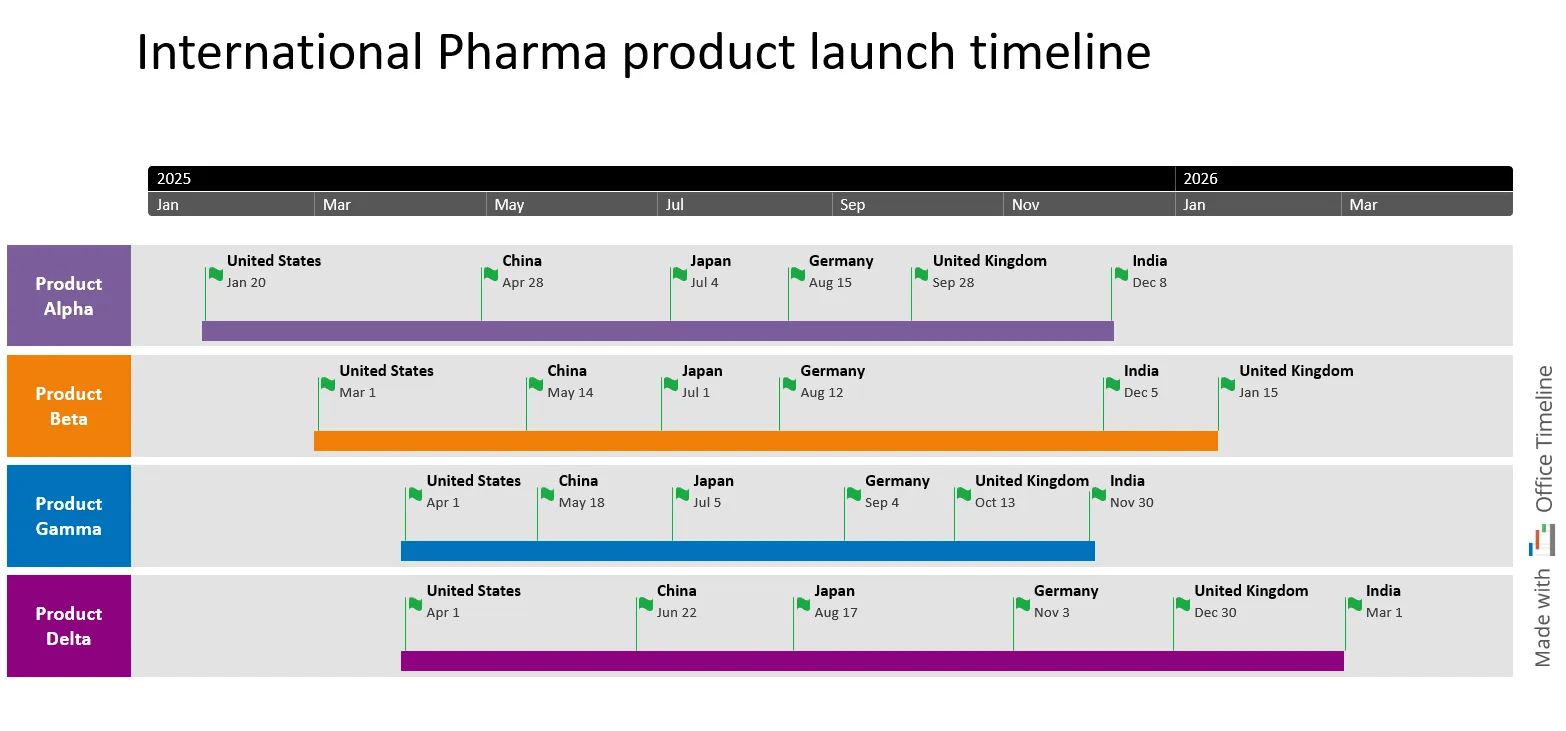
Or picture this scenario: Your company is gearing up for an international launch of several pharmaceutical products, each aimed at key global markets like the United States, China, Japan, Germany, the United Kingdom, and India, with launch dates spread over 15 months.
You need to present this strategic rollout plan at an upcoming pharma conference. You need a clear, engaging way to communicate the complexity of these product launches to stakeholders and industry peers. You decide to use a structured timeline to visually map out your launch strategy:
This timeline highlights each product’s specific launch dates, showing how the schedules overlap and where careful sequencing is necessary to prevent resource strain and regulatory delays. Adding this visual to your presentation makes it easy to show how careful planning can lead to a successful launch.
It demonstrates how your products are prepared for market and how your company can make a stronger impact across regions. At the conference, this timeline can be the starting point for discussions on best practices in global pharma product launches.
With these visuals, you can clearly outline important phases and dependencies, making the information memorable and accessible for your audience. These example timelines are ready-to-use, free, downloadable templates, which can be easily customized as needed. Integrate them directly into your PowerPoint presentation with minimal effort.
The templates were made with the Office Timeline PowerPoint add-in that you can download and try for free for 14 days to make your own timeline from data imported from Excel, MS Project or Smartsheet.
How to make the most of your timelines for pharma projects
We've seen how managing pharmaceutical projects can be more efficient with the use of visual planning tools like timelines. Yet even the best-designed timelines won’t work if they aren’t communicated properly. Regular and clear updates are needed to make sure the information is understood and put into action.
Whether you’re managing projects, tracking clinical trial milestones or presenting complex data at pharma events, consider these best practices to make the most out of your timelines:
Keep it simple
Avoid overloading your timeline with too much detail. Just highlight the most important milestones and dependencies. You can provide additional information in supporting documents if necessary.
Use visual hierarchies
Make sure the most important dates and tasks stand out visually. For this, use colors strategically or vary text sizes.
Customize communication
Consider the needs and preferences of your audience. When presenting to scientific peers at a conference, for example, emphasize the data and research aspects. When updating executives, focus on high-level insights and implications.
Finally, use timelines to break down complex information and be smart about how you present your data. Keeping things visually simple and your message sharp will make these tools work harder for you, making your case memorable and sparking meaningful discussions.
Frequently asked questions
We’ve covered how timelines can help you keep clinical trials on track, or make the most of your resources, or create clear presentations. Now, let’s tackle some common questions about managing and presenting pharmaceutical projects.
Milestones in clinical trials are specific checkpoints that mark the most important stages of the study, such as starting patient recruitment, completing the treatment phase, or finalizing data analysis. These planned, measurable events help track progress, prioritize tasks that need attention, and keep the study on schedule.
The difference between Phase 1, 2, 3, and 4 clinical trials lies in their objectives, from initial safety testing to post-approval monitoring and the way they are managed. If you think of managing a clinical trial like running a project, each phase comes with its own set of management requirements to keep timelines on track and coordinate multiple teams.
Phase 1 trials involve a small group of 20 to 100 people (FDA - The Drug Development Process). The goal is to test safety and establish an appropriate dosage and to monitor side effects. Clinical trial management at this point is directed towards avoiding risks and efficient data collection, given the early stage of development.
Phase 2 expands to 100 to 300 patients to evaluate how well the treatment works for a specific condition; safety monitoring is continued. Phase 2 management goes more towards refining protocols and ensuring patient recruitment timelines are met. Also, changes might occur based on early results, which need coordination.
Phase 3 includes 300 to 3,000 participants and gathers data on effectiveness and adverse reactions. Managing this phase is more complex and requires coordination across multiple sites and strict adherence to regulations. The data collected is used for regulatory submissions, with careful planning needed to align timelines with approval milestones.
Phase 4 starts after the treatment is approved and released to the public. It tracks long-term safety and effectiveness. Management collects real-world data and makes adjustments to comply with safety standards.
In healthcare, resource allocation refers to the process of distributing available resources (for example, funds, medical equipment, and personnel) among various programs, or populations. This distribution occurs at both macro levels (e.g., national health budgets) and micro levels (e.g., hospital departments). The goal is to use these resources efficiently and equitably to improve health outcomes.
A sequential clinical trial allows for data analysis at multiple points during the study rather than waiting until the end. This method lets researchers assess results at predetermined intervals and decide whether to continue, adjust, or stop the trial. It can lead to quicker decisions and more efficient resource use.
Standard pharmaceutical product development takes about 10 to 15 years and involves several stages: discovery and development (3-6 years) identifies potential treatments, followed by preclinical research (1-2 years) using lab and animal studies to evaluate safety. Clinical trials include three phases to test safety and dosage, examine efficacy and confirm results and last 1-4 years. And finally, regulatory review takes 6 months to 2 years for approval, with post-market surveillance to monitor safety.
Here’s an example structure and some practical advice on how to build a medical PowerPoint presentation to clearly present complex medical information:
Title slide
Start with a Title slide that lists the presentation name, your name, and your affiliation so your audience knows who you are and what to expect.
Introduction
Use the Introduction for a quick background and explain why your topic matters, setting a clear purpose.
Objectives
Next, outline the Objectives to give the audience a roadmap of what you’ll cover.
Main content
When you get into the Main content, break it down into manageable sections:
- Methods - explain your methods simply,
- Results - use graphs, charts and timelines in the Results section to make data easy to digest and discuss what your findings mean. Mention limitations as well.
Conclusion
Wrap things up with a Conclusion that highlights the take-home points and suggests next steps if relevant.
References
Don’t forget References to back up your claims and an Acknowledgments slide to thank anyone who contributed.
Questions
Finish with a Questions slide to open up for discussion and connect with your audience. This structure can help make your presentation more engaging and easier to follow.
To create an effective medical PowerPoint presentation, focus on clarity and simplicity. Here are 4 simple suggestions to help you make a good medical PowerPoint:
- Keep your slides clean
- a good medical PowerPoint presentation starts with a well-organized structure;
- minimize text and avoid dense paragraphs;
- bullet points work best for summarizing the main ideas.
- Use high-quality visuals, like graphs, timelines or Gantt charts. Illustrate concepts rather than relying on text-heavy explanations.
- Keep it consistent. Stick to the same font and color scheme throughout to maintain a professional look.
- Engage your audience. Create slides that complement, not just repeat, your spoken content.
Tip: Instead of wasting hours on PowerPoint, use a PowerPoint template to get a head start.
Project management tips and tricks
Turn project data into professional timelines
Get the advanced features of Office Timeline free for 14 days.